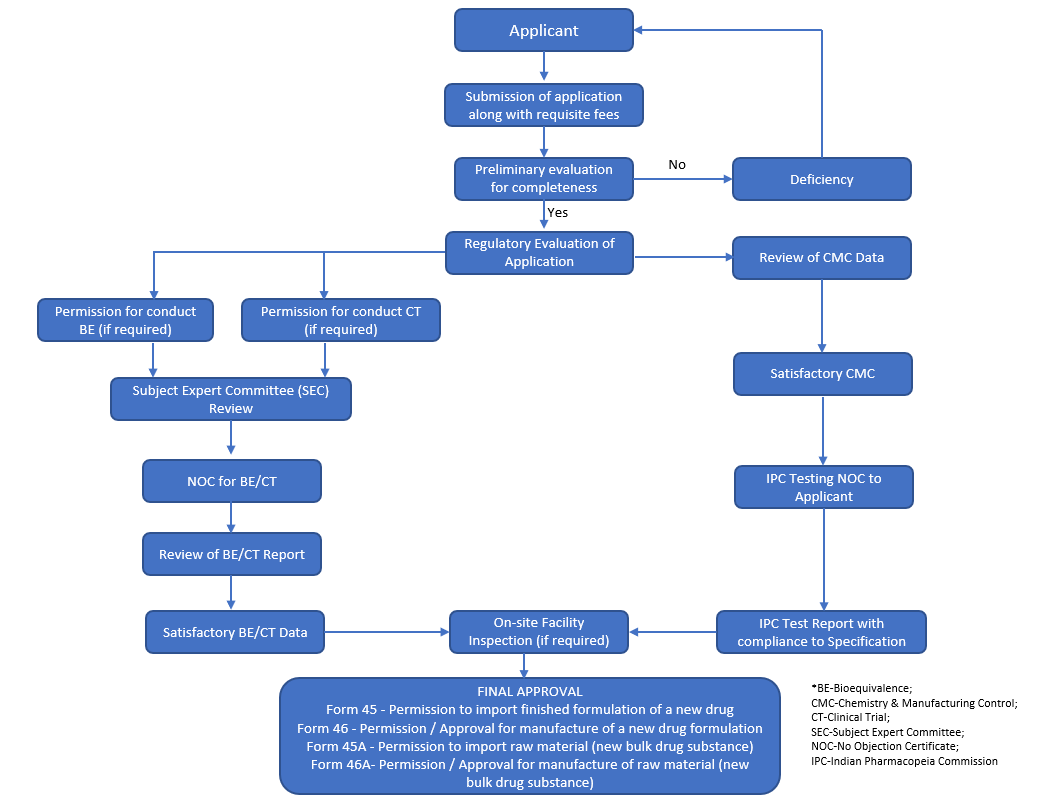
DCG(I) approval process for Drug Substance / Drug Product
India: Frequently asked Question about New Drugs
Question 1: What is considered as a “New drug” or “New Chemical Entity” in India?
Answer: A new drug as define under Rule 122-E of Drugs and Cosmetics Rules means:
- a drug, including active pharmaceutical ingredient or phytopharmaceutical drug, which has not been used in the country to any significant extent has not been approved as safe and efficacious by Central Licencing Authority (CLA) i.e., DCG(I) with respect to its claims; or
- a drug approved by the CLA for certain claims and proposed to be marketed with modified or new claims including indication, route of administration, dosage, and dosage form; or
- a fixed dose combination of two or more drugs, approved by CLA separately for certain claims and proposed to be combined for the first time in a fixed ratio, or where the ratio of ingredients in an approved combination is proposed to be changed with certain claims including indication, route of administration, dosage, and dosage form; or
- a modified or sustained release form of a drug or novel drug delivery system of any drug approved by the Central Licencing Authority; or
- a vaccine, r-DNA derived product, living modified organism, monoclonal antibody, stem cell derived product, gene therapeutic product or xenografts, intended to be used as drug.
Question 2: What is an Investigational New Drug (IND)?
Answer: An Investigational New Drug (IND) means a new chemical or biological entity or substance that has not been approved for marketing as a drug in any country.
Question 3: What is a Subsequent New Drug (SND)?
Answer: A subsequent new drug means a drug approved by the Central Licencing Authority for certain claims and proposed to be marketed with modified or new claims including indication, route of administration, dosage, and dosage form. A subsequent new drug also includes a new drug already approved in the country.
Question 4: Which Regulatory Bodies in the Indian Government are responsible for the registration of NCE?
Answer: The main regulatory body in India is Central Drug Standard Control Organization (CDSCO) under the Ministry of Health and Family Welfare (MoHFW). The Drugs and Cosmetics Act of 1940 regulates the import, manufacture, distribution, sale of drugs and cosmetics in the country.
The Rules 122A, 122B, 122 DAB, 122DAC, 122 DD and 122E of Drugs and Cosmetics Rules and Appendix I- XII of Schedule Y, describe the information/data required for approval of clinical trial and/or to import or manufacture of new drug for marketing in the country.
Question 5: How can one apply for registration and what steps are followed?
Answer: Following are the steps used for Local manufacturing of New Drug: (Refer flowchart at end)
- CT/BE permission from the CDSCO
- New Drug Application (NDA) to the CDSCO
- Application to obtain Manufacturing License from state FDA, state where the manufacturing site exists.
Following are the steps used in case of Import:
- CT/BE permission from the CDSCO
- NDA to the CDSCO
- Import Registration (Form 41) application to the CDSCO
- Import License (Form 10) application to the CDSCO.
Question 6: What are the format requirements, is it CTD, eCTD, ASEAN or some Other?
Answer: In India, as per the CDSCO, CTD (ICH) is followed. CDSCO guidance document, Guidelines on CTD for New Drug Application (NDA) is applied for format requirement. Submit two hard copies and two soft copies i.e., CD’s (PDF format).
Question 7: In India, does electronic submission is essential or is it voluntary only?
Answer: All the applications submitted through SUGAM portal only to CDSCO. Link to SUGUM Portal is https://cdscoonline.gov.in/CDSCO/homepage . User Manual For e-Governance Solution for CDSCO: https://cdsco.gov.in/opencms/export/sites/CDSCO_WEB/Pdf-documents/SUGAM_user_manual.pdf
Question 8: Whether permission from CLA is required to conduct BA/BE study of new drug and investigational new drug in human subjects?
Answer: Yes. Any person or institution or organization who intends to conduct BA/BE study of a new drug or an investigational new drug in human subjects shall obtain permission for conducting the BA/BE study from the CLA by making an application in Form CT05. However, no permission from CLA is required for conduct of any BA/BE study in animals.
Question 9: Timelines for processing of application
| Process | Timeline |
| To conduct BA/BE study in human subjects | 90 working days |
| Time for Regulatory Approval of CTA/IND Application | 16-18 weeks |
| Time for Evaluation of MAA | 8-12 weeks |
Question 10: In case of Import, the data from Foreign Clinical Trials (FCT) is permitted in a Marketing Authorization application?
Answer: Yes, CDSCO may ask for submission on safety and efficacy on Indian patients.
Question 11: Are there requirements to include local clinical trials in a Marketing Authorization Application?
Answer: If FCT is accepted, then there is no need to conduct local CT. But if FCT are rejected or not performed then local Ct are required.
Question 12: What are different rules governing clinical trials?
Answer: Rule 122-A -Application for permission to import new drug
- Rule 122-B -Application for approval to manufacture new drug
- Rule 122-DA-Mandatory requirement of permission from DCG (I) for conduct of clinical trial of new drug.
- Rule 122 DAB -Provision for examination of serious adverse event (SAE) of injury and death and payment of compensation in clinical trial related cases. Provision for debarment of the applicant in case of failure to pay compensation.
- Rule 122 DAC -Conditions of permission for conduct of clinical trial which includes mandatory requirement to follow Good Clinical Practice (GCP) guidelines, guidelines and requirements specified in Schedule Y of Drugs and Cosmetics Rules and other applicable regulations. Provision for debarment of applicant and investigator in case of non- compliance
- Schedule Y-Detailed guidelines and requirements for conduct of clinical trial and approval of new drug
Question 13: Which language is acceptable for application? Is it being necessary to include the National Language? Is the application form being in English or National language?
Answer: In India, only English language is accepted. There is no requirement to include Indian national language in any part of application or on label or package insert of Drug product. The application form is also in English.
Question 14: In Country representative is required or not?
Answer: Yes, the in-country representative is required.
Question 15: Who can be importer or who can file application for grant of permission to manufacture new drugs for sale or distribution?
Answer: A person/organization having a valid drug manufacturing license for any drug issued under the Drugs and Cosmetics Act, 1940 and Rules, 1945 can file an application for grant of permission to manufacture new drugs for sale or distribution.
Question 16: For NDA filing, what are the requirements for stability conditions in India?
Answer: In India, for New Drug Filing (NDA) 12 months long term (30°C/70%RH) stability data and minimum 6 months accelerated stability data is essential, on basis of which 24 months shelf life can be obtained.
Question 17: Does GMP Certification necessary for NDA submission?
Answer: Yes, GMP certification is necessary for NDA submission.
GMP requirements for manufacture of drugs are prescribed in the Schedule M of the D & C Rules.
Question 18: How many samples of drug substance and drug product are required for filing?
Answer: Samples of drug substance and drug product (an equivalent of 50 clinical doses or double the quantity required (whichever is more)) for complete testing of product with testing protocols, full impurity profile and release specifications should be forwarded to Central Drugs Laboratory, as and when required / instructed.
Question 19: Is there any convenience of priority or expedited review in India?
Answer: Yes, there is a priority review available for drugs indicated in life-threatening / serious diseases or diseases of special relevance to the Indian health scenario.
Question 20: What is accelerated approval process for new drugs?
Answer: Accelerated approval process may be allowed to a new drug for a disease or condition, considering its severity, rarity, or prevalence and the availability or lack of alternative treatments, if there is a prima facie case of the product being of meaningful therapeutic benefit over the existing treatment.
- In such case, the approval of the new drug may be based on data generated in clinical trial where surrogate endpoint shall be considered rather than using standard outcome measures such as survival or disease progression, which are reasonably likely to predict clinical benefit, or a clinical endpoint. These should be measurable earlier than irreversible morbidity or mortality (IMM) and reasonably likely to predict clinical benefit.
- After granting accelerated approval for such drug, the post marketing trials shall be required to validate the anticipated clinical benefit.
- Accelerated approval may also be granted to a new drug if it is intended for the treatment of a serious or life-threatening condition or disease of special relevance to the country.
- and addresses unmet medical needs. This provision is intended to facilitate and expedite review of drugs so that an approved product can reach the therapeutic armamentarium expeditiously.
- If the remarkable efficacy is observed with a defined dose in the Phase II clinical trial of investigational new drug for the unmet medical needs of serious and life-threatening diseases in the country, it may be considered for grant of marketing approval by the Central Licencing Authority based on Phase II clinical trial data. In such cases, additional post licensure studies may be required to be conducted after approval to generate the data on larger population to further verify and describe the clinical benefits, as per the protocol approved by the Central Licencing Authority.
- The type of information needed to demonstrate the potential of a drug to address an unmet medical need will depend on the stage of drug development. Early in development, such potential should be sufficiently demonstrated based on nonclinical models, a mechanistic rationale, and pharmacologic data. Later in development, prior to new drug approval such potential should be demonstrated through clinical data to address an unmet medical need.
Question 21: For how much time the certificate is valid?
Answer: For five years.
Question 22: What is procedure to renew the registration or re-registration?
Answer: At the time of application for renewal of registration or re-registration, the application is to be made 9 months before the expiry of the Registration Certificate. Following documents need to be submitted:
- Form 40, POA, GMP / COPP,
- Registration certificate,
- DMF (soft copy if no change),
- License (sale or manufacturing License of drugs of the agent) etc.,
- the undertaking by the manufacturer or his authorized agent in India
- in respect of any administrative action taken due to adverse reaction or
- in respect of any change in manufacturing process, or in packaging, or in labeling or in testing, or in documentation of any of the drug pertaining to this Registration Certificate or
- in respect of any change in the constitution of the firm including name and /or address of the registered office/ factory premises operating under this Registration Certificate.
- Details of drugs imported in India during last three years. v. Submission of original RC issued.
Question 23: Post approval changes- Variations?
Answer: Link to guidance for post approval changes: https://cdsco.gov.in/opencms/export/sites/CDSCO_WEB/Pdf-documents/biologicals/CDSCO-GuidanceForIndustry.pdf
Question 24: Checklists
Answer: For applications for Investigational New Drugs (INDs)
For approvals of a new drug (Formulation) already approved in the country and others.
Link: https://cdsco.gov.in/opencms/resources/UploadCDSCOWeb/2018/UploadIndustryChecklist/checklistSND.pdf

